In 2011, the FDA identified an association of ALCL (anaplastic large cell lymphoma), a type of non-Hodgkin’s lymphoma and a cancer of the immune system, with breast implants. This cancer is known formally as BIA-ALCL and in the majority of cases, has been found in the scar tissue surrounding breast implants or the fluid nearby. The risk of this cancer is very low at approximately 1:30,000 of breast implant placements, although research is ongoing. It is generally assumed to be associated with only textured implants. (However, recently the FDA stated that there have been known cases in women with smooth breast implants.) There have been cases reported with other types of implants as well including those used in orthopedic knee replacement. Generally speaking, current research suggests that some kind of irritation due to the surface of the implant and possible bacterial contamination are contributing factors.
COSMETIC SURGERY TRENDS, NEWS, & INSIGHTS FROM DR. ZUCKERMAN
Weight fluctuations can significantly affect the size and shape of the breasts and may introduce ptosis or sag.
Dr. Zuckerman shares his tips for critiquing breast augmentation before and afters as a plastic surgeon would to help you identify subtle markers of the best surgeons: those with high levels of technical surgical skill and high-quality cosmetic surgery outcomes across large and small augmentations.
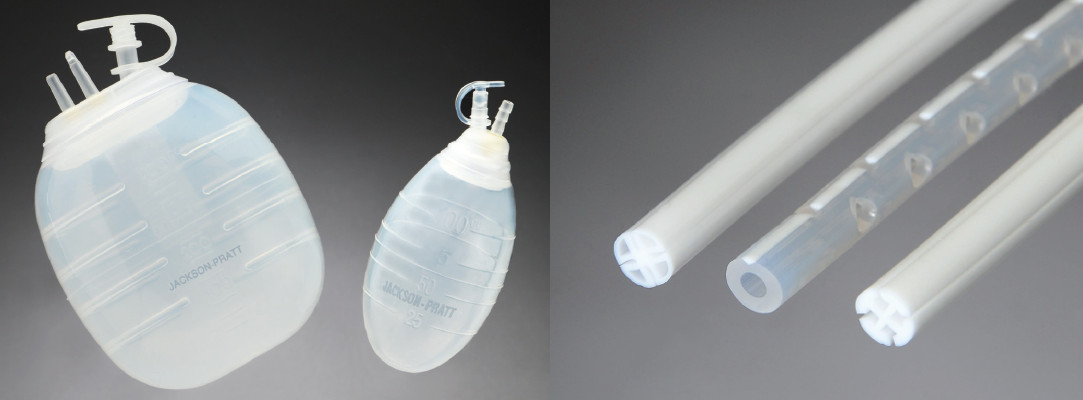
Some plastic surgeons use a technique developed several years ago to perform a tummy tuck without placing surgical drains for postoperative care, or drainless tummy tuck. I describe the technique and also demystify the easy and painless process of maintaining a postoperative surgical drain for a short period of time.
Learn more about capsular contracture, which affects 4-8% of breast augmentation patients. Dr. Zuckerman is familiar with all the latest protocols to avoid bacterial contamination to minimize this risk.
Learn more about the meticulous operative techniques that Dr. Zuckerman employs to minimize bleeding and postoperative discomfort. Among them, he applies a long-acting local anesthetic during surgery to minimize immediate postoperative pain for up to three days. Patients often return to work within a few days.
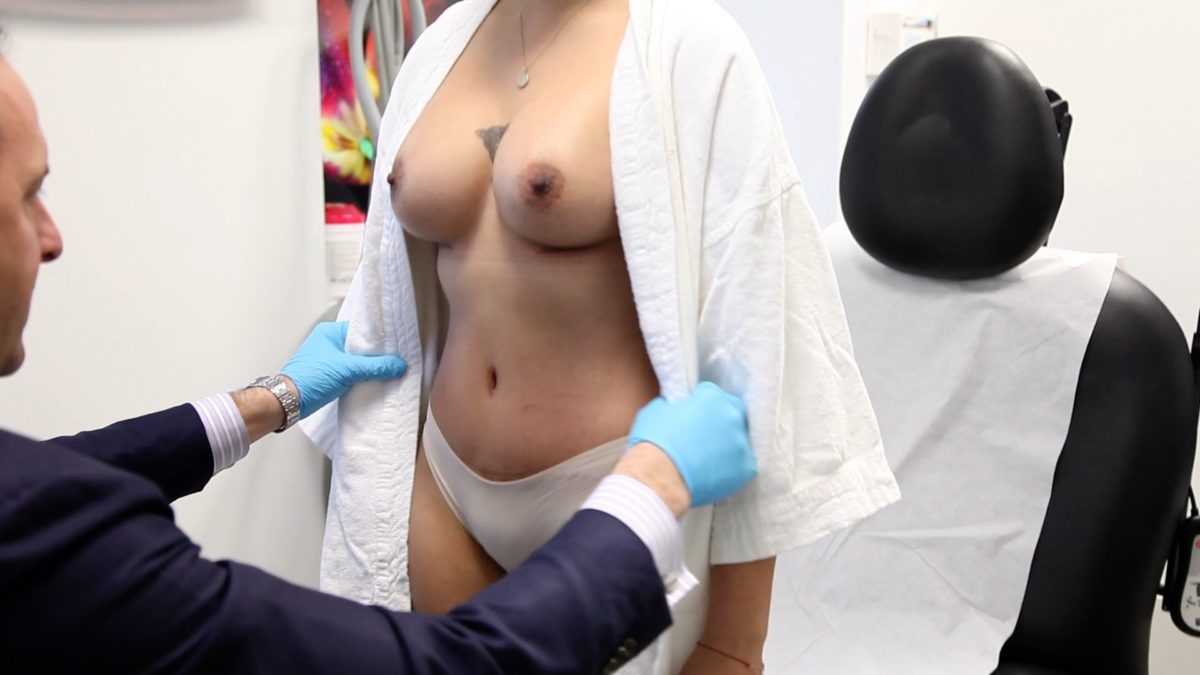
Dr. Zuckerman filmed a patient feature showcasing the cosmetic surgery process. This patient underwent a combined breast augmentation and tummy tuck to reverse the signs of two pregnancies.